During Batch and Continuous Operations in the Real World Th Eadded Nitrogen Source

By Jeff Arthur, Technical Sales Director, Allpax, a ProMach product brand
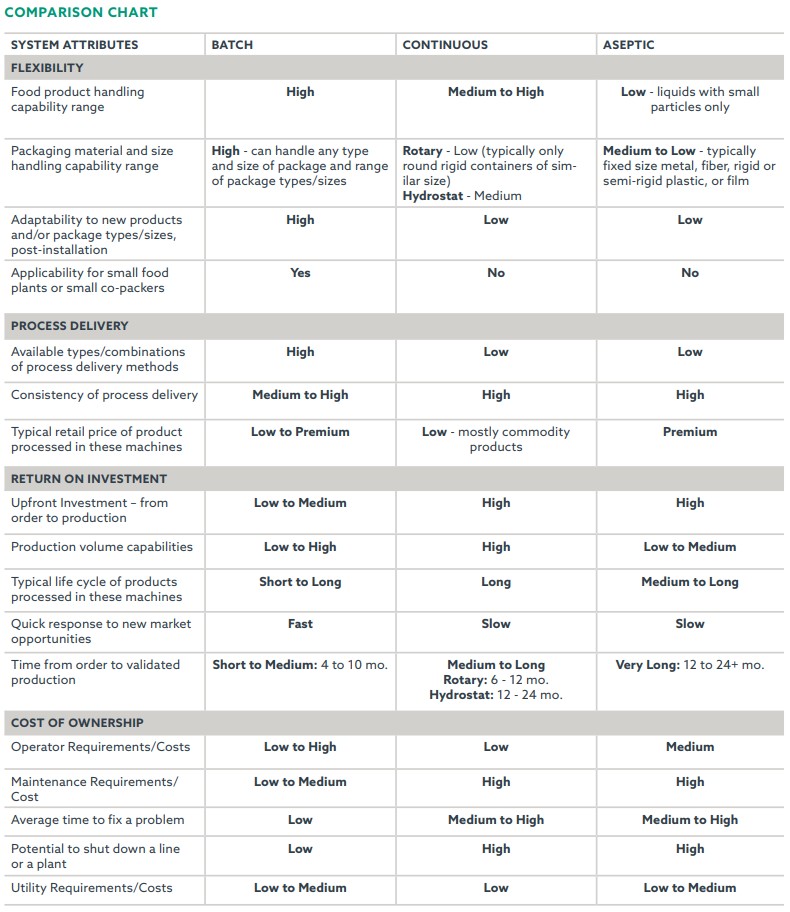
Understanding the typical pros and cons of batch versus continuous and aseptic sterilization of low-acid shelf-stable food and beverage products helps organizations make an informed purchasing decision. Typical Total Cost of Ownership (TCO) factors to consider in the selection process include product type, package type, product/package/process flexibility needs (short and longer term), throughput requirements, product life cycle, price point of the product, time to market requirements, and size of the overall investment both upfront and down the road.
The types of sterilization processes considered here are:
- Batch Retorting/Sterilization
- Continuous Retorting/Sterilization (rotary and hydrostatic)
- Aseptic Processing/Sterilization

Batch Retorts / Sterilizers / Autoclaves
Batch retorts are the most predominant type of in-container, thermal sterilization systems used by food and beverage processors throughout the world today. Batch retorts are, just as the name implies, sterilization vessels that process a "batch" of prepackaged products at a time. Large baskets or trays containing hundreds, or thousands of pounds of low-acid food or beverage products packaged in metal, glass, rigid or semi-rigid plastic, or flexible film are either manually or automatically loaded into these pressure cookers that usually process anywhere from one to twelve or more baskets of products per batch. Typical products processed in batch retorts include pet foods, fruits, vegetables, rice, beans, soups, ready to eat (RTE) meals, meat, fish, baby foods, dairy products, ready to serve beverages and nutraceuticals, and even some pharmaceutical products.
Temperature and pressure in the batch retort are increased and then brought back down in the cooling phase(s) so that the product in the package is rendered commercially sterile, and/ or cooked to the appropriate texture for quality purposes. Pressure inside the vessel can be very tightly controlled during the process cycle so that the package is not deformed or damaged by expansion or contraction during processing. Many different types of thermal process methodologies can be employed by a batch retort, including 100% saturated steam, full or partial water immersion, water spray, water cascade or shower, steam-air, or a combination thereof to provide for the maximum in product and/or package handling flexibility. Different agitation methods, including full or partial rotation (end over end) or horizontal reciprocating action, can also be employed to enhance convective heating and cooling of the product within the package so that the retort cycle time can be reduced, which in turn can help to minimize the degradation of the products from the heat. A premium horizontal reciprocating agitation method that has been developed in recent years is called the SHAKA process. Using the SHAKA process for some product/package combinations significantly decreases the sterilization time to achieve product quality results that rival what can be accomplished in aseptic processing systems.

Batch retort systems offer the most flexibility in terms of the ability to handle an extremely vast range of products and package types and sizes, to employ a variety of process delivery methodologies, to deliver precise temperature and pressure control, and to quickly respond to new market opportunities down the road. In terms of plant production, if a retort is down for scheduled maintenance or emergency repairs the other retorts in the retort room can continue processing, which improves overall line efficiency. Batch sterilization of shelf-stable food and beverages is ideal for co-packers, seasonal operations, and small, medium, and large producers that need to have short- and longer-term product and package flexibility, and/or to accommodate shorter product life cycles.

Batch retorts are relatively simple to operate and maintain compared to continuous machines. Batch retort rooms can be either manually operated with employees loading/ unloading baskets or trays and/or moving the basket/tray stacks into and out of the retorts, or the retort room can be partially or fully automated and operated by a minimum number of personnel (i.e.: ~1 to 2).
A batch retort can be shipped via truck and can be designed, built, installed and placed into full production in about four to six months for just the retort(s), or up to eight to ten months for a completely automated batch retort system.
Laboratory batch retorts can be used to develop new formulation process profiles that can be exported to production batch retorts. Both production and lab retorts are controlled via PCs and/or programmable logic controllers (PLCs) with touchscreen operator interfaces (HMI). Recipes stored in the controller enable the batch retort to change sterilization profiles based on the requirements of the next batch.

Continuous Sterilizers (Rotary and Hydrostatic)
Continuous rotary and hydrostatic sterilizers differ from batch sterilizers in that in-container sterilization is accomplished in a completely automated and continuous fashion. Filled packages leave the steamer or capper and are conveyed directly to the sterilizer where they enter the machine, are sterilized and cooled, and then exit in a continuous fashion.

Continuous rotary sterilizers are mainly limited to handling cylindrical packaging such as round cans, and in some rare cases, glass or plastic jars or bottles. They are typically used to process convective (non-viscous) heating/ cooling products that require or benefit from some amount of agitation, including products like canned fruits or vegetables in juice/water/brine; soups/ready meals; whole, evaporated and flavored milks; infant formula and nutritional drinks, etc. Containers are subjected to intermittent, axial type agitation as they progress through the cooking and cooling vessels. Rotary sterilizer lines require at least two vessels in the system to cook and cool the product, and typically consist of three or more vessels to accommodate the cooking and/or cooling times, and throughput requirements. These machines are ideally suited for a relatively small range of round steel cans (typically only one size) filled with products that have similar cook and cool time requirements, and/or for high throughput applications. Product heat degradation risk is typically low simply because every package travels the exact same path and is exposed to the exact same processing environment throughout the sterilization system.
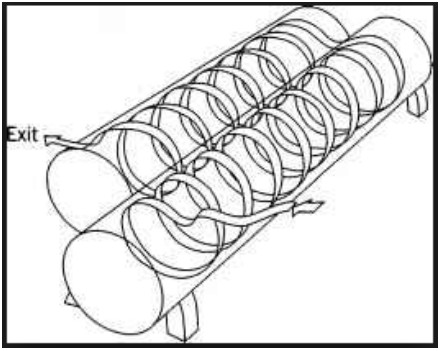
Capital cost is typically high and return on investment (ROI) can be longer than batch retorts due to the commodity pricing on most products that are sterilized through a rotary sterilizer. Total investment ranges from $1.5 up to $5 million These are more complex machines than batch retorts, and therefore can be challenging and costly to maintain. If the continuous rotary sterilizer goes down, the entire line must stop until the sterilizer is fixed. And in most cases, this can result in a total loss of all product in the sterilizer.
Time to market for a rotary sterilizer is typically longer than a batch retort because of its relative complexity. Typical order to production time is 6 to 12 months.

Continuous Hydrostatic Sterilizer
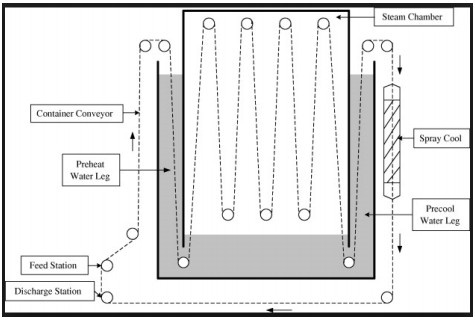
In continuous hydrostatic sterilizers, products that are hermetically sealed in containers are automatically conveyed through multiple heating and cooling towers. These towers can be 60 or more feet tall. A building to house the hydrostatic towers is typically constructed around the machine, especially in cold climates, which can add to the capital cost. Each tower performs a different function of heating and cooling under pressure. Unlike the continuous rotary sterilizers, the hydrostatic sterilizer is typically a static type sterilizer for processing conductive (viscous) heating and cooling products such as pet food, formulated bean products, canned meats/ready meals, condensed soups, baby food, pumpkin, etc. The machine can be designed to process a wider range of container types and sizes. Once built however, the range of product and package types that can be handled in the machine is set. It typically requires a major modification and investment to change over to a significantly different type or size of package that is outside the original design criteria. Continuous hydrostatic sterilizers provide the utmost in economical long run, high volume processing capabilities for product and container combinations that will likely not change over time.
Of the three in-container type sterilizers, the hydrostatic sterilizer requires the highest capital investment, which can be anywhere between $5 and $20 million U.S. dollars. ROI will typically be the longest due to the high capital investment (machine + infrastructure + installation costs) and maintenance requirements.
If the hydrostatic sterilizer goes down due to equipment failure, the lines feeding the sterilizer must stop, and the risk of having to discard an entire machine's product (tons of product) in such cases is high.

Hydrostatic towers are shipped in sections and assembled on site, which is a major construction project undertaking. Control systems are typically more complex than batch retorts or rotary sterilizers. Construction time to market will typically exceed a year, and installation and validation can consume six or more months.
Aseptic Sterilizers
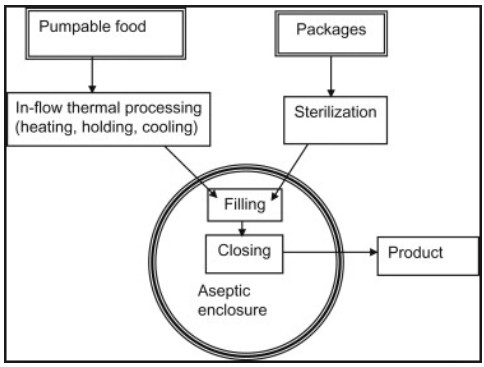
In aseptic sterilization, liquids are sterilized and cooled as they pass through a set of heat exchangers in a continuous process. The product is then piped into pre-sterilized packaging in a clean room environment. Among the types of sterilizers already discussed, aseptic sterilizers cause the least amount of product heat degradation because they heat the product directly and for a very short period of time, versus heating the product inside the package. Near-fresh product quality is maintained, as are nutrient levels, due to the rapid cooking and cooling times achieved. Homogeneous liquid products such as milk, cream, ice cream mix, yogurt, salad dressing, liquid eggs, and fruit juices and concentrates are rendered shelf stable in this process and maintain very high levels of product quality. Albeit more rare, aseptic sterilizers can also accommodate liquids with small discrete particles, such as cottage cheese, baby foods, tomato products, fruit and vegetables, soups, and rice desserts.

However, validation of aseptic systems that handle liquids with particulates can be very difficult, time consuming, and risky to operate. Aseptic packaging for products that are normally refrigerated, such as milk, are useful in third world or in emergency situations where power supply is spotty or nonexistent. Once the line is designed and built, there is little flexibility in the type of product and/or packaging that can be run on the line.
Changeover to a different product involves time consuming clean-in-place processes. Time to market for the installation of these systems is as long or longer than a hydrostatic sterilizer. Validation of the system requires the longest period of time among the different shelf-stable sterilization technologies. Control systems are typically very complex. Operators work in a clean room environment and undergo the highest level of operator training. Maintenance and troubleshooting require highly trained individuals. Aseptically sterilized products typically command premium prices. This fact combined with the high output of these continuous systems means that ROI is typically faster than that for a hydrostatic sterilizer.
Typical total investment for an aseptic sterilization system is in the millions, if not tens of millions of dollars. Aseptic sterilization is an option to explore for organizations with the resources, knowledge, experience, and ability to handle high tech/high volume, clean-room environment type production.
About the author: Jeff Arthur, Technical Sales Director, Allpax, has over 31 years of experience in the industrial food processing equipment industry, primarily associated with the design, manufacturing and sales/customer support of in-container, thermal sterilization/processing equipment.
Source: https://www.retorts.com/white-papers/batch-versus-continuous-and-aseptic-sterilization/